Come together, electrostatically, as one
How do 2 proteins come together to form a complex? Although we can sometimes determine the crystal structure of a complex, this only gives us a static picture, the end product of a dynamic process.
To provide a glimpse into how two proteins come together, Tang and co-workers, in the paper "Visualization of transient encounter complexes in protein–protein association" Nature (2006) 444:383, devised a brilliant scheme to capture the alternative ways that two proteins bind together in solution. They studied the binding of the N-terminal domain of enzyme I (EIN) to the phosphocarrier protein (HPr). The figure on the left shows EIN on the left (mostly blue). HPr is the protein on the right (green). In the experiment, they attached Mn2+ ions (3 red balls on the far right) onto the surface of HPr.
They studied the binding of the N-terminal domain of enzyme I (EIN) to the phosphocarrier protein (HPr). The figure on the left shows EIN on the left (mostly blue). HPr is the protein on the right (green). In the experiment, they attached Mn2+ ions (3 red balls on the far right) onto the surface of HPr.
The key idea is that these Mn2+ ions induce a magnetic response in EIN where the magnetic response (ΔΓ2) of the backbone H atoms in EIN depends on the distance of the H atoms from the Mn2+ ions. The magnetic response ΔΓ2 (red dots) for each backbone H atom in EIN is plotted below: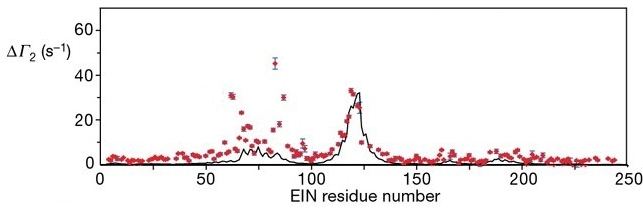
The black line in the figure above is the ΔΓ2 calculated from the crystal structure of the complex of EIN bound to HPr. The measured ΔΓ2 (red dots) show a number of peaks that deviate from the black line. These peaks correspond to transient binding sites of HPr to other parts of the EIN surface.
From the results of a series of similar experiments, Tang and co-workers reconstructed the most likely transient binding sites of HPr to EIN. The alternate conformations of HPr are shown as a density map (green) in the following image. The actual binding site of HPr in the crystal structure is shown in blue, and EIN is shown as an eletrostatic surface map (red is positive, white neutral, blue is negative):
The alternative conformations of HPr bind to positively charged parts of the surface of EIN. However, near the actual binding site, there are few alternate conformations, even though the surface around the actual binding site is also strongly charged.
Tang and co-workers conclude that it is very easy for two proteins to stick together, drawn by simple electrostatic attraction. But this binding is weak. To explain the absence of alternative conformations around the actual binding sites, they argue that once the HPr binds close to the actual binding site, there is a large energy funnel that forces the HPr into the actual binding site.
This is the first study to show, experimentally, that two proteins can transiently bind anywhere through electrostatic interaction, a truly significant result.

2 comments:
I read this paper as well. I thought the analysis was quite crude. It is still questionable how stable these encounter complexes are, or how general this mechanism is.
Hx
I think you're nit-picking. There's 2 parts to the paper, an experimental part, and an analysis part. As far as I am concerned, the experimental part nails the existence of transient states. This has never been done before.
As for the analysis part, this is used to estimate the stability/frequency of the transient binding. Yes it is crude, but I really don't care. The important thing is the experimental curve. I just want an order of magnitude estimate, and to see if there's anything qualitative about the solutions.
Some caveats, it is a nature paper, so many details are left out. This makes the analysis seem rushed. One of the nice things about this study is when you have 3 different experimental curves to refine your data against, you actually have excellent constraints on your monte-carlo simulation. That's why experiment is *always* better than careful simulations.
As for generality, this is never something you can establish in the first paper (unless you're doing mathematics). It's only something that can be decided after many studies.
Post a Comment